
Our new report
 Health Innovation KSS, UK National Innovation Centre for Ageing (NICA), VOICE and Pfizer UK with support from Unity Insights and National Institute for Health and Care Research (NIHR) Applied Research Collaboration Kent Surrey and Sussex (ARC KSS) have developed a new report ‘One size doesn’t fit all: reimagining medicines information for patients.’
Health Innovation KSS, UK National Innovation Centre for Ageing (NICA), VOICE and Pfizer UK with support from Unity Insights and National Institute for Health and Care Research (NIHR) Applied Research Collaboration Kent Surrey and Sussex (ARC KSS) have developed a new report ‘One size doesn’t fit all: reimagining medicines information for patients.’
The new report investigates the current medicines product information paradigm and whether it can be enhanced to more positively influence both patient experience of and adherence with prescription medication. The report also explores if an opportunity exists to improve how this information might be provided in the future using digital solutions with the aim of increasing its value to patients and healthcare professionals (HCPs).
Patients, carers, healthcare professionals (HCPs) and senior healthcare system stakeholders were asked what they think about current medicines product information, and if it could be improved using digital solutions.
Current state of medicines labelling
Medicines labelling and packaging are designed to keep patients safe. Regulators work together with pharmaceutical manufacturers to put into place routine risk minimisation measures for every medicinal product, including a standardised Summary of Product Characteristics (SmPC), the package label and the paper Patient Information Leaflet (PIL) that comes with each package of medicine.
The World Health Organisation (WHO) estimates that between 30-50 per cent of patients do not take medicines according to prescription and product information instructions. Despite this, the product information paradigm for human medicines has not changed substantially for decades. Is it still fulfilling its stated intention? What do patients in the UK think about current product information and how would they like it to look in the future?
Time for change
 Our new report shows that a change is clearly needed. Patients are ready, but this change can’t leave people behind. Understanding of product information can be improved for human medicines using a range of digital solutions. Based on what patients, HCPs and senior stakeholders indicated, the potential result of this improved understanding would be that patients are better able to take their medicines correctly, which in turn could improve their health outcomes and reduce the burden of medication errors on the system.
Our new report shows that a change is clearly needed. Patients are ready, but this change can’t leave people behind. Understanding of product information can be improved for human medicines using a range of digital solutions. Based on what patients, HCPs and senior stakeholders indicated, the potential result of this improved understanding would be that patients are better able to take their medicines correctly, which in turn could improve their health outcomes and reduce the burden of medication errors on the system.
Collaboration between different stakeholders is critical to make this happen – with industry, government, regulators, third sector organisations and academia working together with NHS patients and HCPs. This report proposes bringing these stakeholders together to work in three key areas to develop and implement a UK roadmap for electronic product information (ePI).
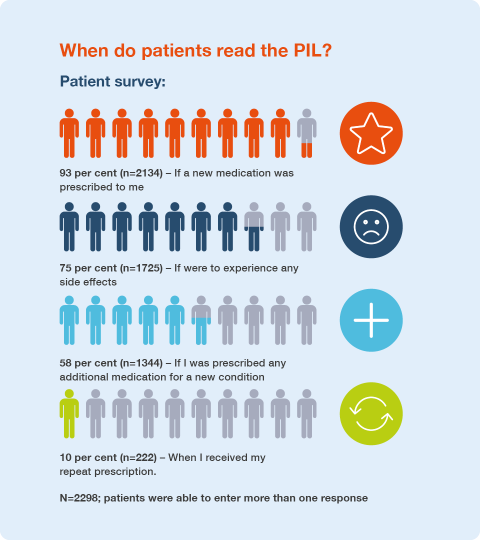
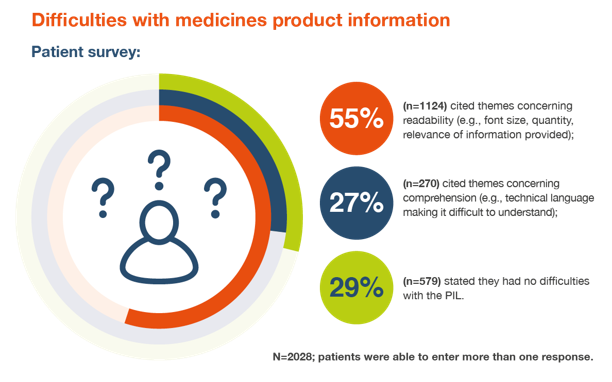
Patient survey findings
Recommendations
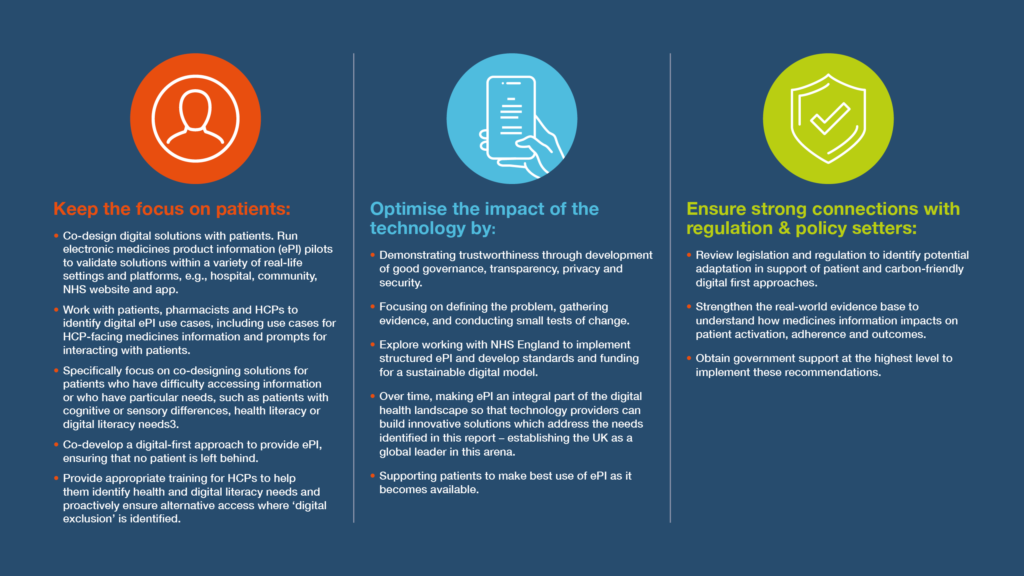
How were these recommendations developed?
These recommendations are based on the analysis of several streams of information. A preliminary literature search was carried out that informed which stakeholders were engaged and the engagement approach. A survey was developed which received almost 3000 responses, and focus groups and interviews were conducted to make sure a diverse range of voices were heard. Analysis of this information gave some key insights that were used to develop the recommendations. These recommendations were reviewed in a forum by stakeholder representatives to assess their credibility, refine them and consider how they could be implemented.

 +44 (0)300 303 8660
+44 (0)300 303 8660
 enquiries@kssahsn.net
enquiries@kssahsn.net
 @kssahsn
@kssahsn